
Description
MechoApedia is a tool dedicated to learning the scientific basis of the mechanisms of toxic action, termed "MechoA" by KREATiS, classified within this scheme. MechoAs are specific molecular initiating events, the first step in Adverse Outcome Pathways, responsible for toxicity to biological organisms of all kinds and therefore these classifications are relevant to both ecotoxicologists and human health specialists (Bauer et al., 2018a, 2018b). This literature review is taken from Bauer’s thesis on MechoAs (Bauer, 2017). Click on MechoAs and their subclasses to get specific information on the interactions between the test substance and the biological matrices.
References:
Adams, W.J., Biddinger, G.R., Robillard, K.A., and Gorsuch, J.W. (1995). A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environmental Toxicology and Chemistry 14, 1569–1574.
Airaksinen, M.M., Rosenberg, P.H., and Tammisto, T. (1970). A Possible Mechanism of Toxicity of Trifluoroethanol and Other Halothane Metabolites. Acta Pharmacologica et Toxicologica 28, 299–304.
ATSDR (1997a). Toxicological Profile: Chloroform.
ATSDR (1997b). Toxicological Profile: Hydrazines.
ATSDR (2014). Medical Management Guidelines (MMGs): Methylene Chloride.
Barron, A.B., Søvik, E., and Cornish, J.L. (2010). The Roles of Dopamine and Related Compounds in Reward-Seeking Behavior Across Animal Phyla. Front. Behav. Neurosci. 4.
Bauer, F. (2017). Une meilleure caractérisation des mécanismes d’action toxique à partir de la structure moléculaire. Université de Haute-Alsace.
Bauer, F.J., Thomas, P.C., Fouchard, S.Y., and Neunlist, S.J.M. (2018a). A new classification algorithm based on mechanisms of action. Computational Toxicology 5, 8–15.
Bauer, F.J., Thomas, P.C., Fouchard, S.Y., and Neunlist, S.J.M. (2018b). High-accuracy prediction of mechanisms of action using structural alerts. Computational Toxicology 7, 36–45.
Berg, J.M., Tymoczko, J.L., and Stryer, L. (2002). A Proton Gradient Powers the Synthesis of ATP. In Biochemistry, (New-York: W.H. Freeman), p.
Blanco-Ayala, T., Andérica-Romero, A.C., and Pedraza-Chaverri, J. (2014). New insights into antioxidant strategies against paraquat toxicity. Free Radical Research 48, 623–640.
Bolton, J.L., Trush, M.A., Penning, T.M., Dryhurst, G., and Monks, T.J. (2000). Role of Quinones in Toxicology. Chemical Research in Toxicology 13, 135–160.
Brown, M.A., and Vito, S.C.D. (1993). Predicting azo dye toxicity. Critical Reviews in Environmental Science and Technology 23, 249–324.
Brüning, T., and Bolt, H.M. (2000). Renal toxicity and carcinogenicity of trichloroethylene: key results, mechanisms, and controversies. Crit. Rev. Toxicol. 30, 253–285.
Calipari, E.S., and Ferris, M.J. (2013). Amphetamine Mechanisms and Actions at the Dopamine Terminal Revisited. J Neurosci 33, 8923–8925.
Casey, J.R., Grinstein, S., and Orlowski, J. (2010). Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11, 50–61.
Chahl, L.A. (1996). Experimental and Clinical Pharmacology: Opioids - mechanisms of action. Australian Prescriber 19, 63–65.
Coats, J.R. (1990). Mechanisms of toxic action and structure-activity relationships for organochlorine and synthetic pyrethroid insecticides. Environ Health Perspect 87, 255–262.
Committee on Acute Exposure Guideline Levels, Committee on Toxicology, Board on Environmental Studies and Toxicology, Division on Earth and Life Studies, and National Research Council (2012). Piperidine. In Acute Exposure Guideline Levels for Selected Airbone Chemicals, (National Academies Press (US)), p.
Dearden, J.C. (2002). Prediction of environmental toxicity and fate using quantitative structure-activity relationships (QSARs). Journal of the Brazilian Chemical Society 13, 754–762.
Di Francesco, A.M., Ward, T.H., and Butler, J. (2004). Diaziridinylbenzoquinones. In Quinones and Quinone Enzymes, (Academic Press), pp. 181–182.
Droual, B. (2011). Chapter 8 - Synaptic Transmission and Neural Integration.
Dunn, M.F., Ramírez-Trujillo, J.A., and Hernández-Lucas, I. (2009). Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 155, 3166–3175.
Elersek, T., and Filipic, M. (2011). Organophosphorus Pesticides - Mechanisms of Their Toxicity. In Pesticides - The Impacts of Pesticides Exposure, M. Stoytcheva, ed. (InTech), p.
Ellison, C.M., Madden, J.C., Cronin, M.T.D., and Enoch, S.J. (2015a). Investigation of the Verhaar scheme for predicting acute aquatic toxicity: Improving predictions obtained from Toxtree ver. 2.6. Chemosphere 139, 146–154.
Ellison, C.M., Madden, J.C., Cronin, M.T.D., and Enoch, S.J. (2015b). Investigation of the Verhaar scheme for predicting acute aquatic toxicity: Improving predictions obtained from Toxtree ver. 2.6. Chemosphere 139, 146–154.
Endo, S., Brown, T.N., and Goss, K.-U. (2013). General Model for Estimating Partition Coefficients to Organisms and Their Tissues Using the Biological Compositions and Polyparameter Linear Free Energy Relationships. Environ. Sci. Technol. 47, 6630–6639.
Enoch, S.J., Hewitt, M., Cronin, M.T.D., Azam, S., and Madden, J.C. (2008). Classification of chemicals according to mechanism of aquatic toxicity: An evaluation of the implementation of the Verhaar scheme in Toxtree. Chemosphere 73, 243–248.
Escher, B.I., and Hermens, J.L.M. (2002). Modes of Action in Ecotoxicology: Their Role in Body Burdens, Species Sensitivity, QSARs, and Mixture Effects. Environ. Sci. Technol. 36, 4201–4217.
Escher, B.I., Hunziker, R., Schwarzenbach, R.P., and Westall, J.C. (1999). Kinetic Model To Describe the Intrinsic Uncoupling Activity of Substituted Phenols in Energy Transducing Membranes. Environ. Sci. Technol. 33, 560–570.
Food and Drug Administration (2004). Docket No. 2004N-0205 Furan in Food, Thermal Treatment; Request for Data and Information.
Franks, N.P., and Lieb, W.R. (1978). Where do general anaesthetics act? Nature 274, 339–342.
Franks, N.P., and Lieb, W.R. (1998). Which molecular targets are most relevant to general anaesthesia? Toxicology Letters 100–101, 1–8.
Fukuto, T.R. (1990). Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87, 245–254.
Grogan, J., DeVito, S.C., Pearlman, R.S., and Korzekwa, K.R. (1992). Modeling cyanide release from nitriles: prediction of cytochrome P 450-mediated acute nitrile toxicity. Chem. Res. Toxicol. 5, 548–552.
IAC Publishing Labs How does caffeine affect plant growth?
Ioannides, C. (1996). The CYP2E Subfamily - Evolution. In Cytochromes P450: Metabolic and Toxicological Aspects, (CRC Press), p. 213.
IPCS Inchem (1998). Diphenylamine (addendum). In JMPR Evaluations, (Rome), p.
IPCS Inchem (2002). Monograph on Atropine - Antidotes for poisoning by organophosphorus pesticides.
Jacobs, A. (1997). Hard and soft nucleophiles. In Understanding Organic Reaction Mechanisms, (Cambridge University Press), pp. 45–46.
Jaworska, J.S., Hunter, R.S., and Schultz, T.W. (1995). Quantitative structure-toxicity relationships and volume fraction analyses for selected esters. Arch. Environ. Contam. Toxicol. 29, 86–93.
Kazius, J., McGuire, R., and Bursi, R. (2005). Derivation and Validation of Toxicophores for Mutagenicity Prediction. J. Med. Chem. 48, 312–320.
King, M.W. (2015a). Ethanol (Alcohol) Metabolism: Acute and Chronic Toxicities.
King, M.W. (2015b). Biochemistry of Neurotransmitters and Nerve Transmission.
Koleva, Y., and Barzilov, I. (2010). Comparative study of mechanism of action of allyl alcohols for different endpoints. 49, 55–59.
Kulma, A., and Szopa, J. (2007). Catecholamines are active compounds in plants. Plant Science 172, 433–440.
Li, X. (2009). Glutathione and Glutathione-S-Transferase in Detoxification Mechanisms. In General, Applied and Systems Toxicology, (John Wiley & Sons, Ltd), p.
Liska, D.J. (1998). The detoxification enzyme systems. Altern Med Rev 3, 187–198.
Lugli, A.K., Yost, C.S., and Kindler, C.H. (2009). Anaesthetic mechanisms: update on the challenge of unravelling the mystery of anaesthesia. Eur J Anaesthesiol 26, 807–820.
McCarthy, M.C., and Enquist, B. (2005). Organismal size, metabolism and the evolution of complexity in metazoans. Evolutionary Ecology Research 7, 681–696.
Moridani, M.Y., Cheon, S.S., Khan, S., and O’Brien, P.J. (2003). Metabolic activation of 3-hydroxyanisole by isolated rat hepatocytes. Chemico-Biological Interactions 142, 317–333.
Munday, R. (1989). Toxicity of thiols and disulphides: Involvement of free-radical species. Free Radical Biology and Medicine 7, 659–673.
Nair, B. (2001). Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Toxicol. 20 Suppl 3, 23–50.
National Center for Biotechnology Information (2017). CONIINE | C8H17N.
Nehlig, A., Daval, J.-L., and Debry, G. (1992). Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Research Reviews 17, 139–170.
O’Donoghue, J.L. (2001). Ketones of Four or Five Carbons. In Patty’s Toxicology, (John Wiley & Sons, Inc.), p.
Olsen, R.W., and DeLorey, T.M. (1999). GABA Receptor Physiology and Pharmacology.
OpenStax (2014). Carbohydrate Metabolism. In Anatomy & Physiology, p.
Petering, D.H., and Fowler, B.A. (1986). Roles of metallothionein and related proteins in metal metabolism and toxicity: problems and perspectives. Environ Health Perspect 65, 217–224.
Recknagel, R.O., Glende Jr., E.A., Dolak, J.A., and Waller, R.L. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacology & Therapeutics 43, 139–154.
van Rensen, J.J.S. (1982). Molecular mechanisms of herbicide action near photosystem II. Physiologia Plantarum 54, 515–521.
Roberts, D.W., Roberts, J.F., Hodges, G., Gutsell, S., Ward, R.S., and Llewellyn, C. (2013). Aquatic toxicity of cationic surfactants to Daphnia magna. SAR and QSAR in Environmental Research 24, 417–427.
Russom, C.L., Bradbury, S.P., Broderius, S.J., Hammermeister, D.E., and Drummond, R.A. (1997). Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry 16, 948–967.
Schultz, T.W. (1987). The use of the ionization constant (pKa) in selecting models of toxicity in phenols. Ecotoxicol. Environ. Saf. 14, 178–183.
Shaw, W. (2002). Chapter 3 Organic Acid Testing, Byproducts of Yeast and their Relationship to Autism. In Biological Treatments for Autism and PDD. Causes and Biomedical Therapies for Autism & PDD., p.
Sherer, T.B., Richardson, J.R., Testa, C.M., Seo, B.B., Panov, A.V., Yagi, T., Matsuno-Yagi, A., Miller, G.W., and Greenamyre, J.T. (2007). Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. Journal of Neurochemistry 100, 1469–1479.
Sikkema, J., de Bont, J.A., and Poolman, B. (1995). Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59, 201–222.
Smith, G.F. (2011). Designing Drugs to Avoid Toxicity. In Progress in Medicinal Chemistry, G. Lawton, and D.R. Witty, eds. (Elsevier), pp. 1–47.
de Sousa, J.P., Torres, R. de A., de Azerêdo, G.A., Figueiredo, R.C.B.Q., Vasconcelos, M.A. da S., and de Souza, E.L. (2012). Carvacrol and 1,8-cineole alone or in combination at sublethal concentrations induce changes in the cell morphology and membrane permeability of Pseudomonas fluorescens in a vegetable-based broth. International Journal of Food Microbiology 158, 9–13.
Vaes, W.H., Ramos, E.U., Verhaar, H.J., and Hermens, J.L. (1998). Acute toxicity of nonpolar versus polar narcosis: Is there a difference? Environmental Toxicology and Chemistry 17, 1380–1384.
Vallabh Minikel, E. (2016). A mechanism of action hypothesis.
Veith, G.D., and Broderius, S.J. (1987). Structure-Toxicity Relationships for Industrial Chemicals Causing Type (II) Narcosis Syndrome. In QSAR in Environmental Toxicology - II, K.L.E. Kaiser, ed. (Springer Netherlands), pp. 385–391.
Verhaar, H.J.M., van Leeuwen, C.J., and Hermens, J.L.M. (1992). Classifying environmental pollutants. Chemosphere 25, 471–491.
Vetter, J. (2004). Poison hemlock (Conium maculatum L.). Food and Chemical Toxicology 42, 1373–1382.
Way, J.L., Leung, P., Cannon, E., Morgan, R., Tamulinas, C., Leong-Way, J., Baxter, L., Nagi, A., and Chui, C. (2007). The Mechanism of Cyanide Intoxication and its Antagonism - Ciba Foundation Symposium 140 - Cyanide Compounds in Biology - Way - Wiley Online Library. (D. Evered and S. Hamett), pp. 232–248.
Wessels, S., and Ingmer, H. (2013). Modes of action of three disinfectant active substances: A review. Regulatory Toxicology and Pharmacology 67, 456–467.
Wiley-VCH Verlag (2002a). Organic peroxides [MAK Value Documentation, 1992]. In The MAK-Collection for Occupational Health and Safety, (Wiley-VCH Verlag GmbH & Co. KGaA), p.
Wiley-VCH Verlag (2002b). Triethylene glycol [MAK Value Documentation, 2007]. In The MAK-Collection for Occupational Health and Safety, (Wiley-VCH Verlag GmbH & Co. KGaA), p.

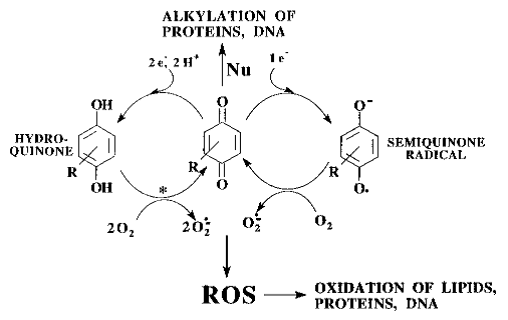
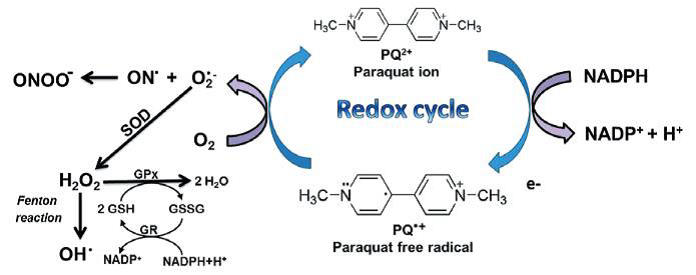 Figure: paraquat RedOx cycle (Blanco-Ayala et al., 2014).
Figure: paraquat RedOx cycle (Blanco-Ayala et al., 2014).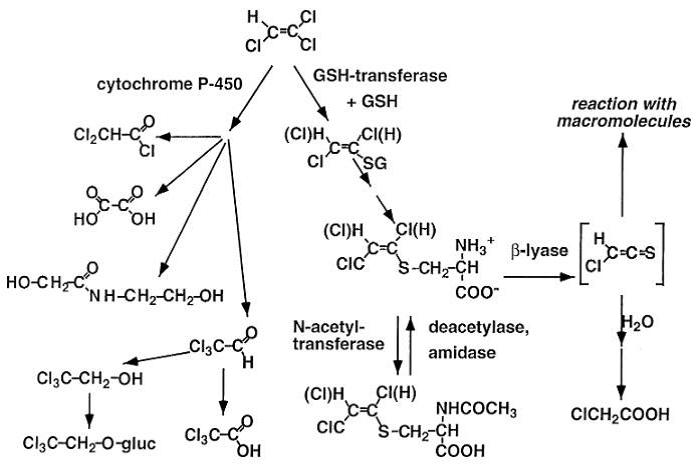

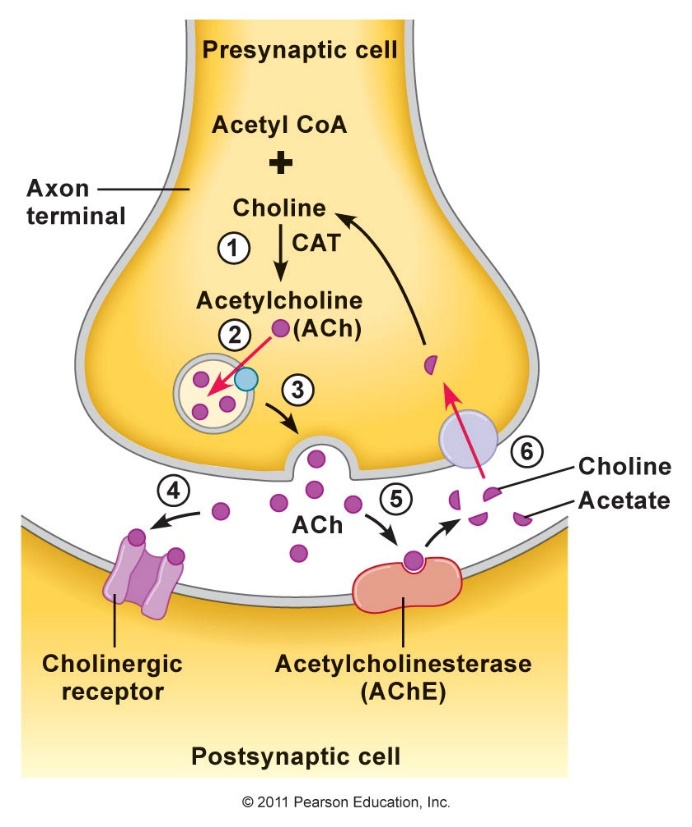
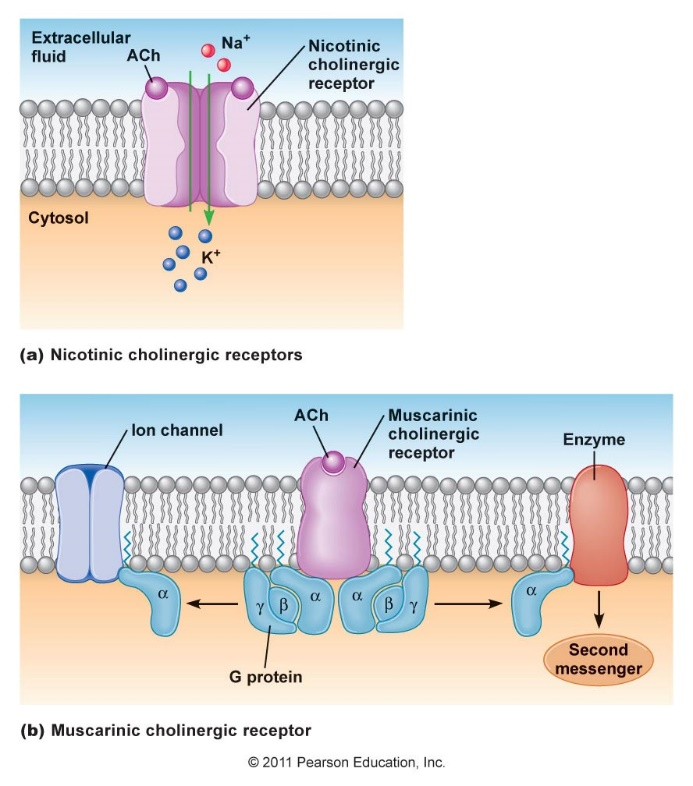
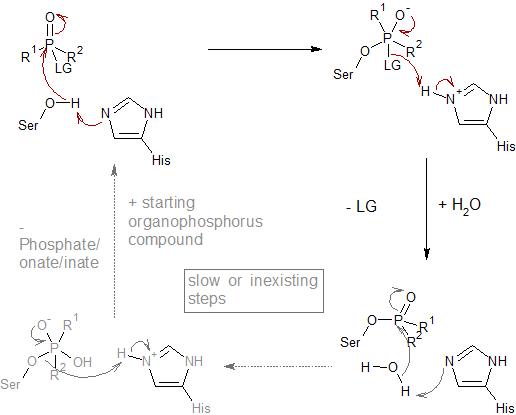
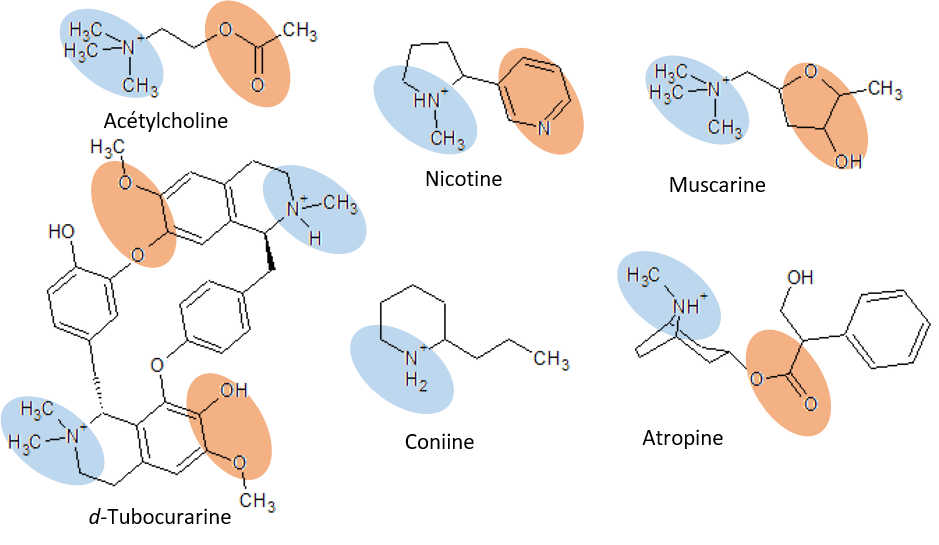 Figure: Molecular structures of acetylcholine and some agonists and antagonists.
Figure: Molecular structures of acetylcholine and some agonists and antagonists.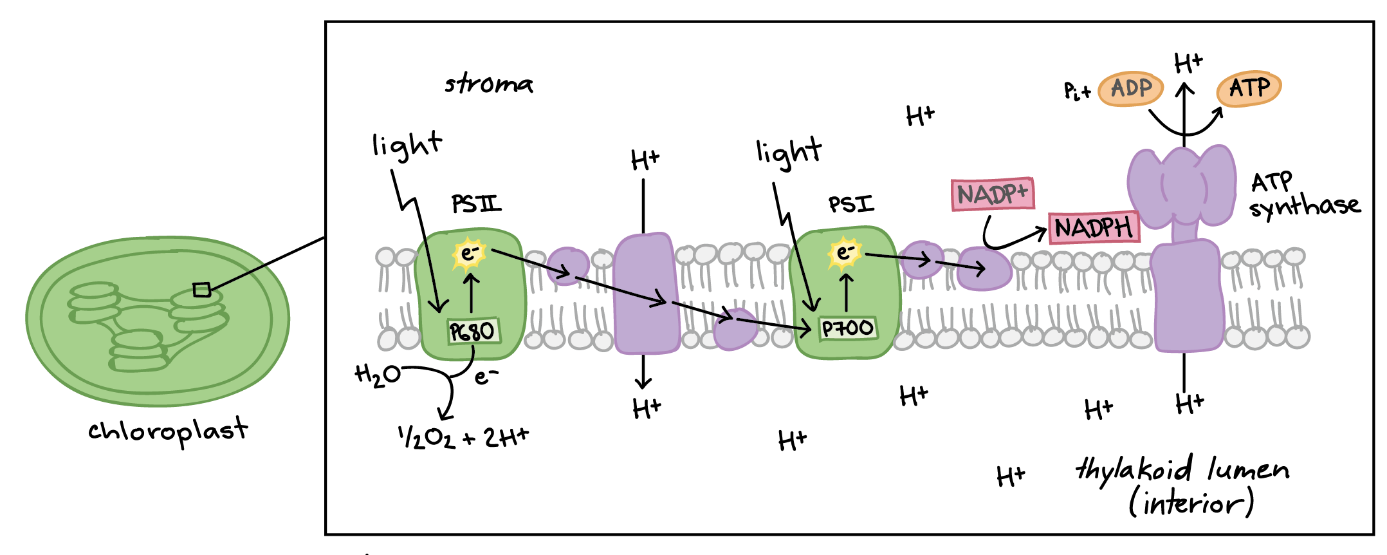
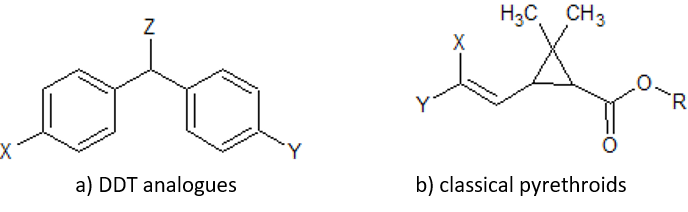
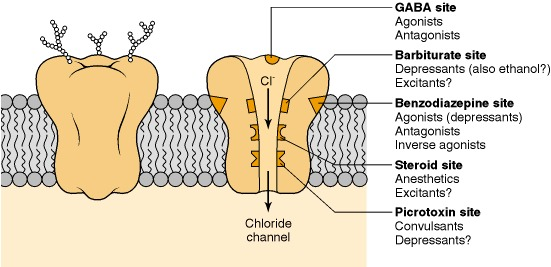
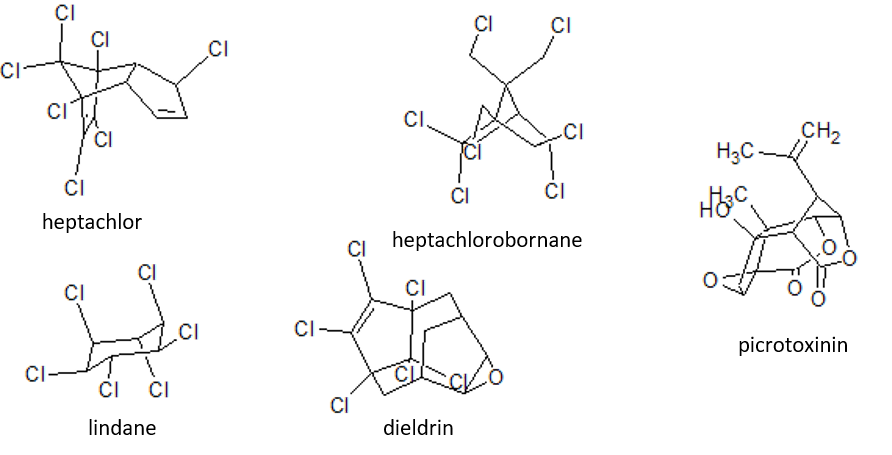 Figure: chlorinated alicyclic insecticides and picrotoxinin.
Figure: chlorinated alicyclic insecticides and picrotoxinin.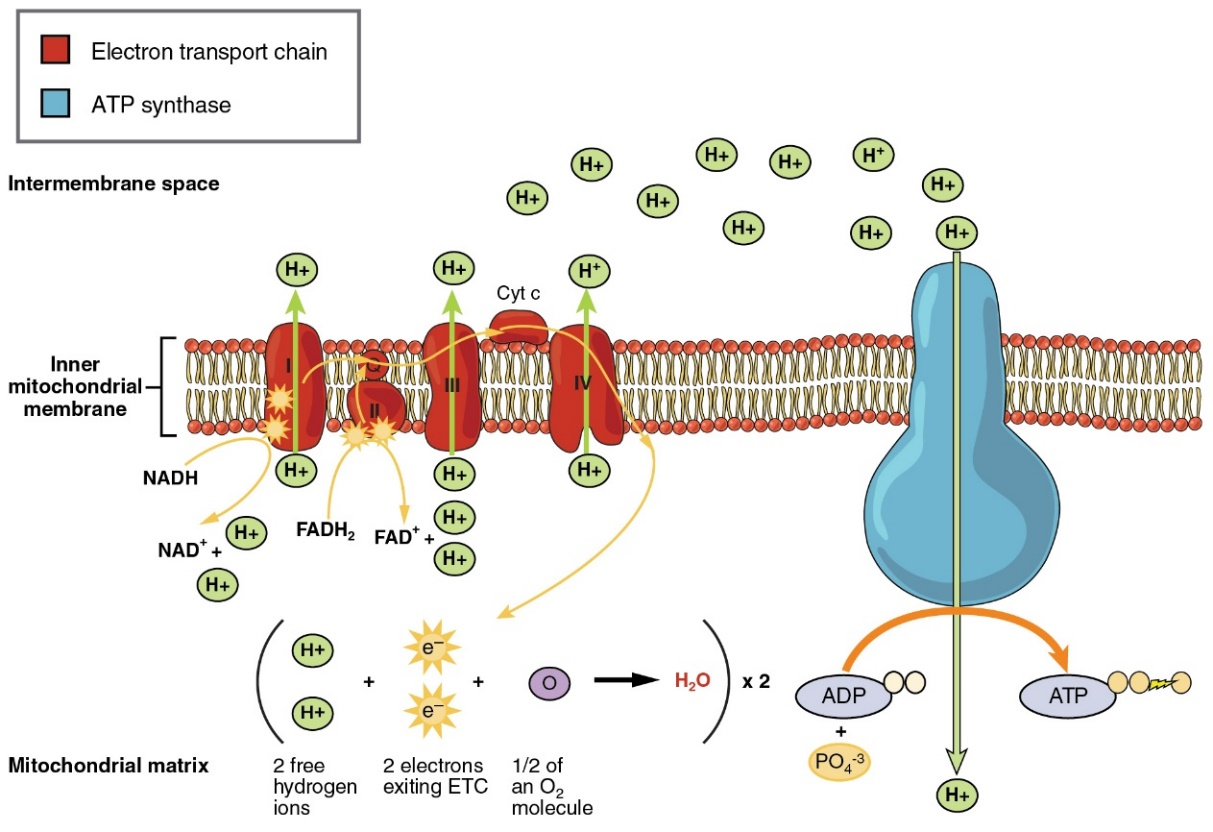 Figure: Coupling of ATP synthase and the electron transport chain with the proton gradient. Taken from Anatomy & Physiology (OpenStax, 2014).
Figure: Coupling of ATP synthase and the electron transport chain with the proton gradient. Taken from Anatomy & Physiology (OpenStax, 2014).