Your in silico expert
We offer our services to screen the hazard of your substance, complete your regulatory dossier, boost your R&D and reduce tests on animals in the most time and cost efficient way possible.
We exist to understand the mechanistic interactions between chemical and biological matrices and quantify the impact on human health and the environment.
La French Touch by KREATiS
We are proud to be one of the first French company to work for the 3Rs, with members from different companies who fight to re-think experimentation.
4 pillars of our activity
The combination of our unique tools will help you to fill your datagaps with the best in silico study predictions available, meeting the 5 OECD principles for the validation of QSARs and regulatory format requirements for a host of dossier submissions and updates notably for REACH and CPSR, ICH M7, Medical devices, but increasingly for aspects of Plant Protection Products, Biocides and Pharmaceuticals.
In silico consulting
Support with Regulatory documents iSafeRat® & for third-party tools for endpoints requiered in dossier submissions.
The power of KREATiS in silico predictions
0
Vertebrate animals saved by the work of KREATiS using in silico methods instead of animal experimentation.
Estimation based uniquely on the number of acute and chronic fish toxicity studies realized by KREATiS from 01/2014 replacing 42 fish in acute OECD 203 and 80 fish eggs in chronic OCDE 210 in vivo studies.

The expertise of model developers
Simply obtaining a prediction is the easy part but having confidence in the reliability of the result requires experience in modelling using multidisciplinary approaches, in silico good practice and unbiased scientific and mechanistic interpretation.
We take pride in sharing our knowledge with you to help you obtain information for your regulatory needs, thanks to our homegrown tools.
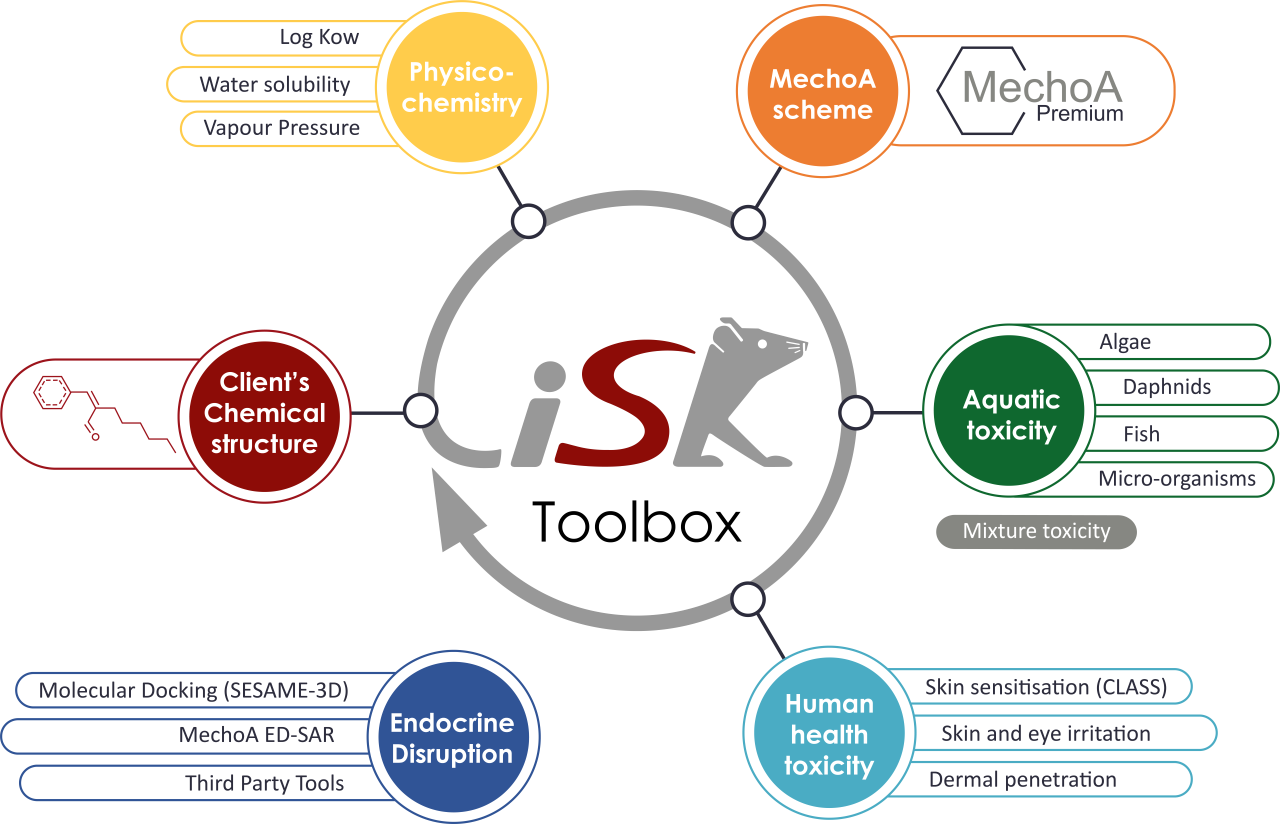
iSafeRat® toolbox
iSafeRat® (in silico Algorithms For Environmental Risk And Toxicity) is the KREATiS-made toolbox of High Accuracy QSAR (HA-QSAR) modules produced by KREATiS to replace all kinds of experimental studies related to human health and the environment.
MechoA
MechoA (Mechanisms of toxic Action) MechoA is a structural alerts-based decision tree to predict the mechanisms of toxic action of a large range of chemical structures in different biological species.
It has been developed in collaboration with Unilever and Liverpool John Moores University, becoming MechoA+, which is made freely available in the OECD QSAR Toolbox.
MechoApedia
Open the door to a clear scientific explanation and better understanding of the mechanisms of toxicity action. KREATiS has designed this interactive pedagogic free tool presenting the different MechoA classes.
Endpoints predictions
Our experts offer expert advice based on our in-house software iSafeRat® and third party software packages to predict toxicology, ecotoxicology and physical chemistry endpoints for all kinds of regulatory and R&D needs.
Physicochemical endpoints
- Octanol/Water partition coefficient (log Kow)
- Water solubility
- Vapour pressure
- Henry’s law constant estimation
Ecotoxicological endpoints
- Acute fish toxicity
- Acute daphnid toxicity
- Algae toxicity
- Chronic fish toxicity
- Chronic daphnid toxicity
- Aquatic toxicity for mixtures
- Toxicity to microorganisms
Toxicological endpoints
- Skin irritation
- Eye irritation
- Skin sensitisation
- Dermal absorption
- Endocrine Disruption potential (ED SAR, SESAME-3D and Third-party tools)
Become a computational specialist
With KREATiS intensive and hands-on training, master your skills and learn how to use software dedicated to predictions to grow your own expertise. The quality of our training is certified each year by the French Certification Qaliopy.
OECD QSAR Toolbox training
An introduction to replacing experimentation including hands on experience using the OECD toolbox for read-across and QSAR modelling.
Read moreIn silico ecotoxicology training
An introduction to regulatory and in silico ecotoxicological principles and methods for predicting hazard and fate without resorting to experimental studies.
Read moreIn silico toxicology training
An introduction to regulatory and in silico toxicological principles and methods for predicting human health effects without resorting to experimental studies.
Read more
What they say about us
The following entities have honoured us by allowing us to quote them on our website.
No quote has been included without the express permission of the person cited and their legal entity.