iSafeRat® toolbox
in Silico algorithms for environmental Risk and toxicity
iSafeRat® is the High Accuracy QSAR (HA-QSAR) platform produced by KREATiS. Initially designed to fulfill endpoints of regulatory dossiers to meet the REACH requirements, iSafeRat® aims to be the most reliable and accurate in silico approach to replace all kinds of experimental studies related to human health, physico-chemistry et environment.
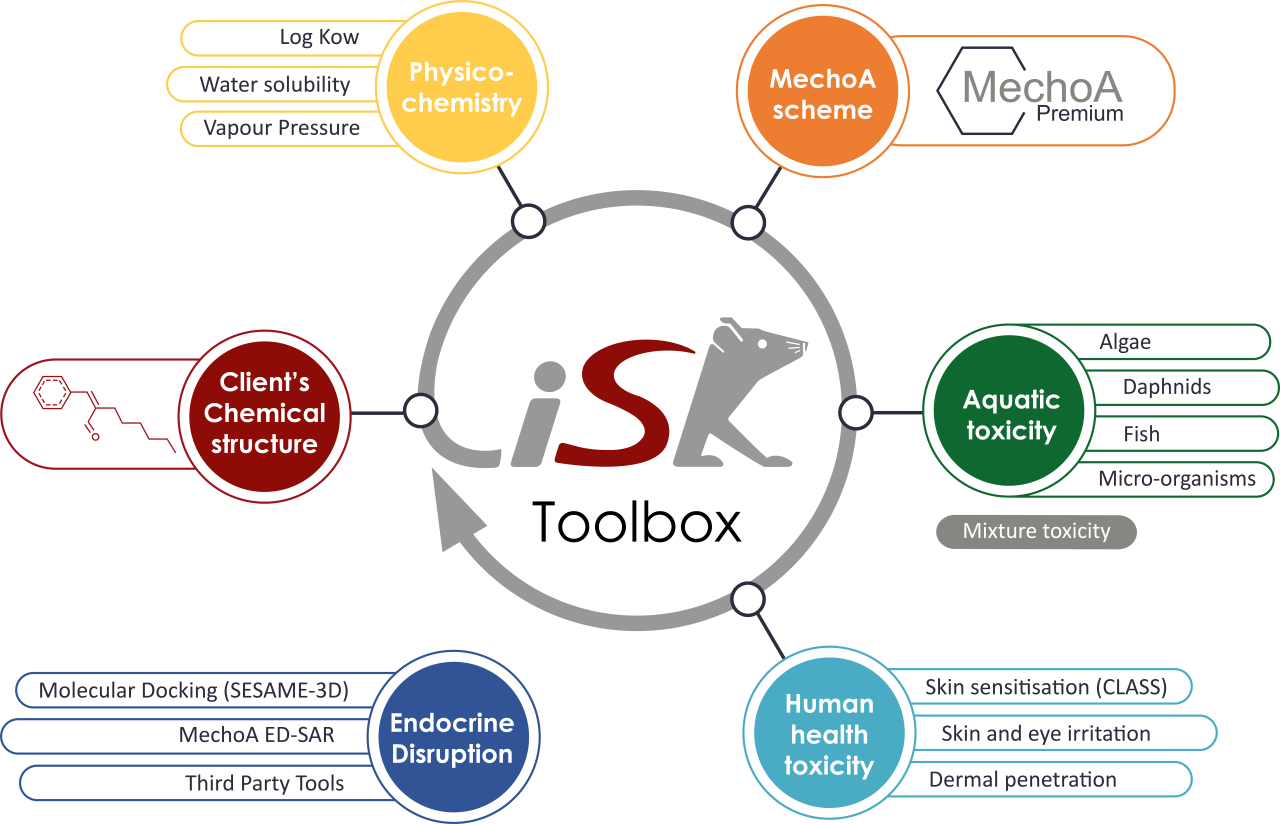
What's in the iSafeRat® Toolbox ?
At KREATiS we believe in our models, and together we can find accurate answers to your questions without resorting to unnecessary experimentation.
Our objective
To provide a fully validated endpoint value which is at least as accurate as that of the best experimental technique available for that endpoint following OECD Guideline methods but for a fraction of the price of a laboratory study and in a fraction of the time.
Each endpoint value that you order for successful regulatory submission will include a full QSAR Model Report (QMRF) and QSAR Prediction Report (QPRF).
SMILES
iSafeRat® uses coded chemical structures (SMILES : Simplified Molecular Input Line Entry System) of organic chemicals to:
- Predict critical physico-chemical parameters
- Define structural alerts within the chemical structure and fit them within a highly structured, published classification scheme (Mechanisms of Toxic Action or MechoAs)
- Quantify their (eco)toxicity from the physico-chemical parameters and the MechoA
Desktop version
iSafeRat® is available as a downloadable desktop version. The predictions come with:
- Applicability domain information* to immediately provide upfront information on the regulatory acceptance status of the prediction
- Confidence interval calculations allowing you to judge the model performance
*3 options are proposed, “inside applicability domain”, “outside applicability domain” or “extrapolated”. “Extrapolated” means we believe in the result but statistically it is not currently completely within domain. The prediction can still be very useful and may be submitted to competent authorities as a weight of evidence.
Models on request
Besides our intention to cover standard regulatory endpoints, KREATiS also offers tailor-made in silico services to meet your specific requirements.
Just as our existing HA-QSARs have been developed and validated (following the latest regulatory guidelines notably R6 and QAF), each of our tailored models are treated with the same level of attention to detail, data verification and statistical model validation.
Depending on your request, we can also prepare a validation report to justify the robustness and, if possible, a mechanistic interpretation for your models.
Endpoints currently available
Octanol/Water partition coefficient (log KOW)
iSafeRat® KOW C2SM study can test the hydrophobicity (as log KOW) of a substance
Study/studies replaced and value reported:
- OECD 107 (Partition coefficient (n-octanol/water): Shake flask method)
- OECD 117 (Partition coefficient (n-octanol/water): High Performance Liquid Chromatography (HPLC) method)
- OECD 123 (Partition coefficient (n-octanol/water): Slow-stirring method
Domain:
- structural space: organic chemicals with H, C, N, O, P, S, F, Cl, Br, I
- response space: -3.77 to 7.00 (but reliable extrapolations can be justified beyond these values)
Methodology:
- From structure: Core-Centred Substitution Approach where the log KOW is calculated by summation of the contributions of all fragments of the molecule.
- Or from high quality water solubility and/or ecotoxicity data provided by the client: Holistic Approach
Accuracy:
As accurate as an OECD 107 or 123 study
Water solubility
iSafeRat® WatSol study can test the water solubility (in mg/L, at 25°C) of a substance
Study/studies replaced and value reported:
- OECD 105 (Water solubility: Shake flask method)
- modified OECD 105 (Water solubility: slow stirring method)
Domain:
- structural space: organic chemicals with H, C, N, O, P, S, F, Cl (and some with Br and I)
- descriptor space: log KOW ranging from -4.45 to 8.43
- response space: water solubility from 0.02 µmol/L to 2000 mol/L (but reliable extrapolations can be justified beyond these values)
Methodology:
- From log KOW: a set of simple linear regressions between Water Solubility and log KOW
- from high quality ecotoxicity data provided by the client (Holistic Approach)
Accuracy:
As accurate as a modified OECD 105 slow stirring study (Thomas & Burosse, 2012, see downloads)
95%-Confidence Intervals available
Vapour pressure
iSafeRat® VP study can test the vapour pressure (in Pa, at 25°C) of a substance
Study/studies replaced and value reported:
- OECD 104 (Vapour Pressure)
Domain:
- structural space: organic chemicals with H, C, N, O, S, F, Cl, Br, I
- descriptor space: boiling point ranging from 26 to 365 °C
- response space: vapour pressure from 10-3 Pa to 105 Pa (but reliable extrapolations can be justified beyond these values)
Methodology:
- a set of simple linear regressions between Vapour Pressure and Boiling Point
Accuracy:
As accurate as an OECD 104 study
95%-Confidence Intervals available
Acute Fish toxicity
iSafeRat® fishLC50 study can accurately predict 96h-acute toxicity to fish (in mg/L) effectively replacing Guideline studies for multiple types of structural groups
Study/studies replaced and value reported:
- OECD 203 (Fish Acute Toxicity Test)
- OECD 236 (Fish Embryo Acute Toxicity (FET) Test)
Domain:
Currently, the ecotoxicity module of the iSafeRat® QSAR models can reliably predict the acute toxicity to fish for chemicals with the following mechanisms of toxic action (MechoA):
- Non-polar narcosis (MechoA 1.1)
- Polar narcosis of alkyl-/alkoxy-phenols (MechoA 1.2)
- Polar narcosis of aliphatic amines (MechoA 1.2 & 5.2)
- Mono-/poly-esters whose hydrolysis products are narcotics (MechoA 2.1)
- Hard electrophile reactivity (MechoA 3.1)
- Soft-electrophile reactivity (MechoA 3.2)
- Pro-reactivity (MechoA 4.3)
- RedOx cycling of primary thiols (MechoA 4.4)
- Proton release of carboxylic acids (MechoA 5.2)
- Aniline like (MechoA an4.3 & 1.2)
for substances with a log KOW between 0 and ca. 5 (and possibly higher), i.e. the solubility/toxicity cut-off point at which acute toxicity is no longer observed below the limit of solubility.
Methodology:
- simple linear regression between subcooled liquid water solubility and toxicity (Mackay et al., 2009; Thomas et al., 2015; Thomas et al., 2018)
Accuracy:
As accurate as an experimental OECD 203 study (in terms of finding true toxicity)
95%-Confidence Intervals
See QMRF for details
Acute daphnid toxicity
iSafeRat® daphEC50 study can acurately predict experimental Guideline 48h-acute toxicity to daphnids (in mg/L) based on immobilisation for wide range of structural groups
Study/studies replaced and value reported:
- OECD 202 (Daphnia sp., Acute Immobilisation Test)
Domain:
Currently, the ecotoxicity module of the iSafeRat® HA-QSAR models can reliably predict the acute toxicity to daphnids for chemicals with the following mechanisms of toxic action (MechoA):
- non-polar narcosis (MechoA 1.1)
- polar narcosis of alkyl-/alkoxy-phenols (MechoA 1.2)
- polar narcosis of aliphatic amines (MechoA 1.2 & 5.2)
- cationic narcosis of quaternary ammoniums (MechoA 1.3)
- mono-/poly-esters whose hydrolysis products are narcotics (MechoA 2.1)
- hard electrophile reactivity (MechoA 3.1)
- Soft-electrophile reactivity (MechoA 3.2)
- RedOx cycling of primary thiols (MechoA 4.4)
- proton release of carboxylic acids (MechoA 5.2)
for substances with a log KOW between 0 and ca. 5 (and possibly higher), i.e. the solubility/toxicity cut-off point at which acute toxicity is no longer observed below the limit of solubility.
Methodology:
- simple linear regression between subcooled liquid solubility and immobilisation of daphnids (Mackay et al. 2009, Thomas et al. 2015)
Accuracy:
As accurate as an experimental OECD 202 study (in terms of finding true toxicity)
95%-Confidence Intervals
Algae toxicity
iSafeRat® algEC50 and iSafeRat® algNOEC studies can test 72h-toxicity (as inhibition of growth) on algae (in mg/L) of a substance
Study/studies replaced and value reported:
- OECD 201 (Freshwater Alga and Cyanobacteria, Growth Inhibition Test)
Domain:
Currently, the ecotoxicity module of the iSafeRat® HA-QSAR models can reliably predict the toxicity to algae (AlgErC10 and AlgErC50) for chemicals with the following mechanisms of action of toxicity (MechoA):
- non-polar narcosis (MechoA 1.1)
- polar narcosis of alkyl-/alkoxy-phenols (MechoA 1.2)*
- polar narcosis of aliphatic amines (MechoA 1.2 & 5.2)*
- cationic narcosis of quaternary ammoniums (MechoA 1.3)*
- mono-/poly-esters whose hydrolysis products are narcotics (MechoA 2.1)
- hard electrophile reactivity (MechoA 3.1)
Soft-electrophile reactivity (MechoA 3.2)
- RedOx cycling of primary thiols (MechoA 4.4)*
- proton release of carboxylic acids (MechoA 5.2)*
for substances with a log KOW between 0 and ca. 5 (and possibly higher), i.e. the solubility/toxicity cut-off point at which acute toxicity is no longer observed below the limit of solubility.
Note: iSafeRat® algNOEC is only available for compounds with MechoA 1.1, 2.1, 3.1 and 3.2
*Currently only available for AlgErC50 predictions
Methodology:
- simple linear regression with subcooled liquid water solubility (Mackay et al. 2009, Thomas et al. 2015)
Accuracy:
As accurate as an experimental OECD 201 study (in terms of finding true toxicity)
95%-Confidence Intervals
Note: In certain studies on algae the test substance may be lost due to metabolisation, volatility of the test substance or adsorption by the algal cells. The iSafeRat® value will equate to the ErC50 (or NOEC) value obtained in a study where the substance is maintained over the whole study period whether or not this can be achieved experimentally.
Chronic fish toxicity
iSafeRat® fishEC10 study can accurately predict the experiemntal result obtained from a Guideline 32d-chronic toxicity on fish (in mg/L) for multiple structural groups
Note: The iSafeRat® fishEC10 study will provide a calculated EC10 value (QSAR based on the most senstive endpoints*: growth/overall survival further to a 32-day study on fish using measured concentrations).
*internal study based on validated data
Study/studies replaced and value reported:
- OECD 210 (Fish, Early-life Stage Toxicity Test)
Domain:
Currently, the ecotoxicity module of the iSafeRat® HA-QSAR models can reliably predict the chronic toxicity to fish for chemicals with the following mechanisms of action of toxicity (MechoA):
- non-polar narcosis (MechoA 1.1)
- polar narcosis of several groups incl. alkyl-/alkoxy-phenols and sulphonates (MechoA 1.2)
- mono-/poly-esters (incl. lactones) whose hydrolysis products are narcotics (MechoA 2.1)
- hard electrophile reactivity (MechoA 3.1)
for substances with a log KOW between 0 and ca. 5.5 (and higher for certain MechoA sub-classes), .i.e. the solubility/toxicity cut-off point at which chronic toxicity is no longer observed below the limit of solubility.
Methodology:
- simple linear regression with subcooled liquid water solubility (Mackay et al., 2009; Thomas et al., 2015)
Accuracy:
As accurate as a well run experimental OECD 210 study (in terms of finding true toxicity)
95%-Confidence Intervals (see QMRF for details)
Chronic daphnid toxicity
iSafeRat® daphEC10 study can predict 21d-chronic toxicity to daphnids (in mg/L) of a substance
Note: The iSafeRat® daphEC10 study will provide a calculated EC10 value (QSAR based on the most senstive endpoint*: reproduction effects further to a 21-day study on daphnids using measured concentrations).
*internal study on validated data
Study/studies replaced and value reported:
- OECD 211 (Daphnia magna Reproduction Test)
Domain:
Currently, the ecotoxicity module of the iSafeRat® HA-QSAR models can reliably predict the chronic toxicity to daphnids for chemicals with the following mechanisms of action of toxicity (MechoA):- non-polar narcosis (MechoA 1.1)
- polar narcosis of alkyl-/alkoxy-phenols (MechoA 1.2)
- mono-/poly-esters whose hydrolysis products are narcotics (MechoA 2.1)
- hard electrophile reactivity (MechoA 3.1)
- Soft-electrophile reactivity (MechoA 3.2)
- proton release of carboxylic acids (MechoA 5.2)
with log KOW between 0 and ca. 6 (and possibly higher), i.e. the point at which toxicity is no longer found below the limit of solubility.
Methodology:
- simple linear regression with subcooled liquid water solubility (Mackay et al. 2009, Thomas et al. 2015)
Accuracy:
As accurate as an OECD 211 study (in terms of finding true toxicity)
95%-Confidence Intervals
Aquatic toxicity for mixtures
As opposed to the other High Accuracy-QSAR models this is a multistep calculation method requiring knowledge of the toxicity properties of each constituent. These can initially be calculated using one of the above ecotoxicity acute modules. Currently KREATiS can accurately predict mixture toxicity (e.g. Natural Complex Substances) for up to 29 constituents. Even if your mixture contains more, it may be possible to get a high quality prediction based on the major constituents and those expected to have the highest toxicity.
iSafeRat® fishLL50 WAF, daphEL50 WAF or algErL50 WAF study can test the acute toxicity on fish, daphnid or agae (in mg/L) of the Water Accommodated Fraction (WAF) of a substance (expressed as the lethal or effective loading rate, L/EL50, in mg/L)
Note. The calculation method is also available to get the chronic result (based on the loading rate once again).
Study/studies replaced and value reported:
- OECD 203 (Fish Acute Toxicity Test)
- OECD 210 (Fish, Early-life Stage Toxicity Test)
- OECD 236 (Fish Embryo Acute Toxicity (FET) Test)
- OECD 202 (Daphnia sp., Acute Immobilisation Test)
- OECD 211 (Daphnia magna Reproduction Test)
- OECD 201 (Freshwater Alga and Cyanobacteria, Growth Inhibition Test)
using WAF method (following OECD Guidance No. 23 Guidance on Aquatic testing of difficult substances and mixtures)
Domain:
- mixture consituents should share the same Mechanism of Action (MechoA), ideally MechoA 1.1 (non-polar narcotics)
- with log KOW between 0 and ca. 5 (and possibly higher), i.e. the point at which toxicity is no longer found below the limit of solubility
Methodology:
The toxicity of mixtuures to aquatic organisms was determined using a calculation method based on toxic additivity principle. That means the toxic parts of each constituent are added up. Therefore the constituents considered within the mixture should act with a similar MechoA. To maximise accuracy of the prediction, the concentration of each constituent in the mixture should be known and is used as inputs into the model.
- First a thermodynamic approach is used to calculate the influence of each constituent on the solubility of the others allowing the concentration of each constituent within the mixture to be determined (i.e. providing the “loading rate”).
- In the second step, the non-bioavailable fraction is removed to determine the true concentration of each constituent exerting toxicity.
- Then, the bioavailable concentrations of each constituent is converted into chemical activity prior to be summed.
- Finally, the loading rate is adjusted until the value of the sum of activities of the bioavailable fractions is equal to the fraction-weighted average of toxic activity of each constituent. This can be seen as a mechanistic description of the process which occurs in an experimental WAF study.
This method has been validated internally on a dozen of Natural Complex Substances for acute exposure with non-polar narcosis constituents. The methodology will soon be available in literature (Bicherel and Thomas, in prep).
Accuracy:
As accurate as a WAF experimental study following the specific guideline
(no confidence limits available)
Toxicity to microorganisms
iSafeRat® asritEC50 study can test the toxicity to microorganisms of activated sludge (as EC50) of a substance
Study/studies replaced and value reported:
- OECD 209 (Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation))
Domain:
Currently, the ecotoxicity module of the iSafeRat® HA-QSAR models can reliably predict the toxicity to microorganisms for chemicals with the following mechanisms of action of toxicity (MechoA):
- non-polar narcosis (MechoA 1.1)
- mono-/poly-esters whose hydrolysis products are narcotics (MechoA 2.1)
with log KOW between 0 and ca. 3 (and possibly higher), i.e. the point at which toxicity is no longer found below the limit of solubility
Methodology:
- simple linear regression with subcooled liquid water solubility (Mackay et al., 2009; Thomas et al., 2015)
Accuracy:
As accurate as an OECD 209 study (in terms of finding true toxicity)
95%-Confidence Intervals
Skin irritation
iSafeRat® iSafeRabbit Skin study can predict the irritant potential to the skin of a substance
Study/studies replaced and value reported:
- OECD 404 (Acute Dermal Irritation/Corrosion)
- OECD 431 & 439 (in vitro)
- Corrosive to skin, Category 1
- Irritant to skin, Category 2
- Non-irritant to skin, No category
Domain:
- Structural domain: organic chemicals with H, C, N, O, S (and some halogenated compounds)
- Physico-chemical parameters domain: molecular weight, vapour pressure, water solubility and Log KOW
- Mechanistic domain: cytotoxicity leading to the clinical signs of irritation/corrosion is considered to be related to the Mechanisms of toxic Action (MechoA)
Methodology:
The iSafeRat Skin Irritation/Corrosion prediction model determines whether the applied dose of a chemical substance causes cytotoxicity, thereby responsible for erythema and/or oedema as both lesions are driving the classification according to CLP and GHS. The dose applied and the physico-chemical properties of the test substance are the input of a series of algorithms to determine the substance concentration in the viable epidermis to establish if this concentration reaches a cytotoxic concentration for the keratinocytes.
Accuracy:
Goodness-of-fit & predictive performance: Accuracy: 84%
Predictive capacity for:
- Corrosives (Cat. 1): 92%
- Irritants (Cat. 2.): 69%
- No category (NC): 88%
Eye irritation
iSafeRat® iSafeRabbit Eye study can predict the irritant potential to the eyes of a substance
Study/studies replaced and value reported:
- OECD 405 (Acute Eye Irritation/Corrosion)
- Corrosive to eye, Category 1
- Irritant to eye, Category 2
- Non-irritant to eye, No category
Domain:
- Structural domain: organic chemicals with H, C, N, O, S (and some halogenated compounds)
- Physico-chemical parameters domain: molecular weight, vapour pressure, water solubility and Log KOW
- Mechanistic domain: cytotoxicity leading to the clinical signs of irritation/corrosion is considered to be related to the Mechanisms of toxic Action (MechoA)
Methodology:
The iSafeRat Eye Irritation/Corrosion prediction model determines whether the applied dose of a chemical substance causes cytotoxicity, thereby responsible for corneal opacity and/or conjunctival redness as both lesions are driving the classification according to CLP and GHS. The dose applied and the physico-chemical properties of the test substance are the input of a series of algorithms to determine the substance concentration in the viable epidermis to establish if this concentration reaches a cytotoxic concentration in the eye tissue.
Skin sensitisation
iSafeRat® CLASS (Classification & Labelling Assessment for Skin Sensitisation) v1.5 model can predict the skin sensitization potential of a substance as assessed by the Local Lymph Node Assay.
Study/studies replaced and value reported:
- OECD 429 (Skin Sensitisation: Local Lymph Node Assay)
OECD 492A & 492B (in vitro)
Skin sensitiser
Not skin sensitiser
Not skin sensitiser (surfactant properties, risk of +ve in LLNA studies only)
Domain:
- Descriptor domain: molecular weight and Log KOW
- Structural domain: organic chemicals with H, C, N, O, S (and some halogenated compounds), fragments of the test item should be found in the training set of the model
- Mechanistic domain: based on MIE, 3 submodels can predict if the substance can undergo autoxidation, can penetrate the skin, can form a reactive metabolite and protein adducts based on iSafeRat® MechoA.
An additional analogue search is implemented to allow the user to have better confidence in the predictions.
Methodology:
The innovative, mechanistic model iSafeRat® CLASS (Classification & Labelling Assessment for Skin Sensitisation) is designed to predict skin sensitisation of organic chemical substances. It is based on Local Lymph Node Assay (LLNA) conducted according to the OECD Guidelines 429, 442A and 442B (OECD, 2010a; OECD, 2010b; OECD, 2018).
The model is an expert-based decision tree generating a final outcome result based on the results of four independent sub-models:
· Hapten/prohapten detection (based on MechoA) v1.2 (hereafter referred as Hapt v1.2)
· Autoxidation (prehapten detection) v2.0 (hereafter referred as AutOx v2.0)
· Skin penetration v1.0 (hereafter referred as SkinAbs v2.0)
· Expected LLNA positive LLNA+ v1.1 (hereafter referred as LLNA+ v1.1)
These sub-models are either based on structural alerts or physicochemical property thresholds.
Skin sensitisation is predicted through 3 sub-models: pre-hapten detection through autoxidation (AutOx v2.0), skin penetration (SkinAbs v2.0), hapten & pro-hapten detection (Hapt v1.2). Additional information is provided based on the results of LLNA+ v1.1. Based on those, a final outcome is given to predict skin sensitisation potency of the substance.
Accuracy:
For iSafeRat® CLASS v1.5 in iSafeRat Desktop v5.2.5 performance of the overall training set of 838 substances (training set and internal validation set) and the external validation set of 237 substances, is:
Dermal absorption
Endocrine Disruption potential (ED SAR)
Study/studies replaced and value reported:
The studies replaced by this 2D SAR include:- Reporter gene assay (RA)
- Enzyme activity assay (inhibition)
Domain:
Currently, this is the list of endpoints that are covered by this model:- Estrogen Receptor α (ESR1) agonism and antagonism
- Estrogen Receptor β (ESR2) agonism and antagonism
- Androgen Receptor (AR) agonism and antagonism
- Thyroid Hormone Receptor α and β (THRA and THRB) agonism and antagonism
- Thyroperoxidase (TPO) inhibition
- Iodothyronine Deiodinase type I, II and III (DIO1, DIO2 and DIO3) inhibition
- Sodium-Iodide Symporter (SLC5A5) inhibition
- Thyroid Stimulating Hormone Receptor (TSHR) agonism and antagonism
- Progesterone Receptor (PGR) agonism and antagonism
- Follicle-Stimulating Hormone Receptor (FSHR) antagonism
- Aromatase (CYP19A1) inhibition
Methodology:
- Curation and thorough validation of in vitro assays (receptor binding, receptor (in)activation, enzyme inhibition) data from various sources, covering more than 8000 substances.
- Grouping of chemicals with common structural patterns and assay outcomes to create structural alerts.
Accuracy:
Each structural alert is evaluated independently for its accuracy. All alerts obtained at least 85% accuracy, however most (95%) alerts reached accuracy >99%, on the training data set. External validation is in progress.Endocrine Disruption Potential (SESAME-3D)
- Docking tools
Study/studies replaced and value reported:
The studies replaced by the workflow include:- Protein binding (Binder/Non Binder)
- Relative binding affinity (RBA, percentage of test item binding to a receptor relative to its natural ligand)
Domain:
Currently, this is the list of endpoints that are covered by this model:- Thyroid Receptor α (THRA) binding
- Thyroid Receptor β (THRB) binding
- Estrogen Receptor α (ESR1) binding
- Estrogen Receptor β (ESR2) binding
- Progesterone Receptor (PGR) binding
- Androgen Receptor (AR) binding
The chemical domain supported for the test molecule covers any type of organic chemicals, and is applicable to all of the listed biological endpoints.
Methodology:
- To date SESAME-3D provides a molecular docking capability for the estimation of the possibility of existence of molecular complexes. In the future a technique called simulation of molecular dynamics will be included as an option for more thorough investigation.
- The molecular docking workflow uses as docking targets only experimentally derived models of the ligand binding domains of the proteins of interest. Thus, the ensemble of experimental models available (from external sources) is curated and clustered into conformational classes, a single representative of which is used as self-standing protein target in a docking experiment.
- Machine learning algorithms are further trained to calculate predicted affinities for the tested and the reference molecules.
- The likelihood of a tested molecule to be a binder is derived from a comparison with the binding profiles of computed molecular complexes for known actives docked within a related biological target.
Accuracy:
The confidence level for each prediction is provided through the report and the interpretation of the results of various statistical tests implemented in the SESAME-3D workflow.The overall accuracy of SESAME-3D is currently under investigation as this workflow is quite a new tool but is anticipated to predict the affinity of native hormones-receptor complexes listed above as studied biological domain, within ±10 nM from the AC50 (concentration providing 50% of maximum effect) reported from in vitro experiments in a consortium database.)
Third-party tools for endocrine disruption
Currently, we use dozens of selected models that have been chosen to make sure we get the most reliable results to predict endocrine disruption potential of chemicals via Estrogenic, Androgenic, Thyroidal (EAT) and non-EATS modalities. We don’t just apply the models to the molecule and report the results. Each model is systematically challenged to make sure it is fit for purpose and a consensus approach is used to arrive at a meaningful conclusion.Study/studies replaced and value reported:
The studies replaced by third party tools include:- Protein binding (Binder/Non Binder)
- Relative binding affinity (RBA, percentage of test item binding to a receptor relative to its natural ligand)
- Transactivation assays (RA)
Domain:
For the following list of endpoints:- Estrogen Receptor α and β (ESR1 and ESR2) binding, agonism and antagonism
- Androgen Receptor (AR) agonism and antagonism
- Thyroid Hormone Receptor α (THRA) binding
- Thyroid Hormone Receptor β (THRB) binding
- Pregnane X Receptor (NR1I2) binding
The applicability domain of each model is different and therefore it is assessed on a case-by-case basis. In general, it covers organic chemicals.
Methodology:
- The in silico models available within the following software packages are currently used to predict endpoints related to ED potential: OECD QSAR Toolbox, Danish QSAR Database, VEGA and T.E.S.T. from US EPA.
- Available third-party models are used to generate the predictions for each test item, followed by a manual reliability check of each prediction, i.e., relative to the prediction based on the models’ goodness-of-fit and/or performance and relative to the test item’s structure (when possible). This step allows to “turn OFF” low reliability alerts and therefore, to reach a conclusion based on only reliable predictions.
Accuracy:
Goodness-of-fit & Performance:Depending on each model used (Reliability cut-off was set at 70% for sensitivity or specificity).
Endpoints in pipeline
Physico-chemical properties
- Log Kow: extension of applicability domain and development of another model based on machine learning (iSafeRat® ALPS)
- Solubility: extension of applicability domain
- Vapour Pressure: extension of applicability domain
Environmental properties
Domain:
The iSafeRat BCF model is based on critical body burden methodology. It is currently a worst case pilot version which has value in providing accurate BCF values only when no metabolism is expected and for highly hydrophylic (e.g. log Kow <2) and hydrophobic (e.g. log Kow >8) substances. The structural domain has currently been limited to providing predictions for MechoA 1.1 substances. Work is in progress to extend the applicability domain of the model for substances expected to metabolise.Methodology:
The model has been created using the Critical Body Burden Hypothesis:Ecotoxicity endpoints
KREATiS is continuously retrieving data to develop new in silico models and to improve the applicability domain of existing ones in ecotoxicology.
Among others, here are the currently on-going tasks:
- update of the acute and chonic toxicity to fish, daphnids and algae for non-polar narcotic compounds (i.e. MechoA 1.1) taking into account main categories of colorants such as anthraquinones, sulfonates, azo and nitro compounds
- update of the acute and chonic toxicity to fish, daphnids and algae for polar narcotic compounds (i.e. MechoA 1.2) taking into account anilines
- update of the acute and chronic toxicity to fish, daphnids and algae for reactive compounds (i.e. MechoA 3.1 and 3.2) like aldehydes, epoxides and acrylates
- new model of the chronic toxicity to fish, daphnids and algae for carboxylic acids (i.e. MechoA 5.2)
Human Health Endpoints
KREATiS is currently working on development of new in silico models to predict these following endpoints in toxicology:
- Sensitisation MIE key event 1, with or without metabolic activation
- Dermal absorption
Endocrine Disruption Potential
KREATiS is currently working on development of new in silico models to predict these following endpoints :
- Molecular Dynamics
- Further development of ED SAR and SESAME-3D as a part of an NC3Rs project.
The endpoint you're looking for isn't already available ?
Get in touch with the KREATiS team today to benefit from our tailored services. Let us know your requirements and one of our team members will contact you to discuss it further.
Contact us