
Our toxicologist Marie Darracq--Ghitalla-Ciock is waiting for you at EUROTOX2024 in Copenhagen
Marie is presenting a poster on the latest update of our model "CLASS" for skin sensitisation now meeting the rigorous requirements of the QSAR Assessment Framework (QAF).
POSTED 2024-09-09
MORE DETAILS
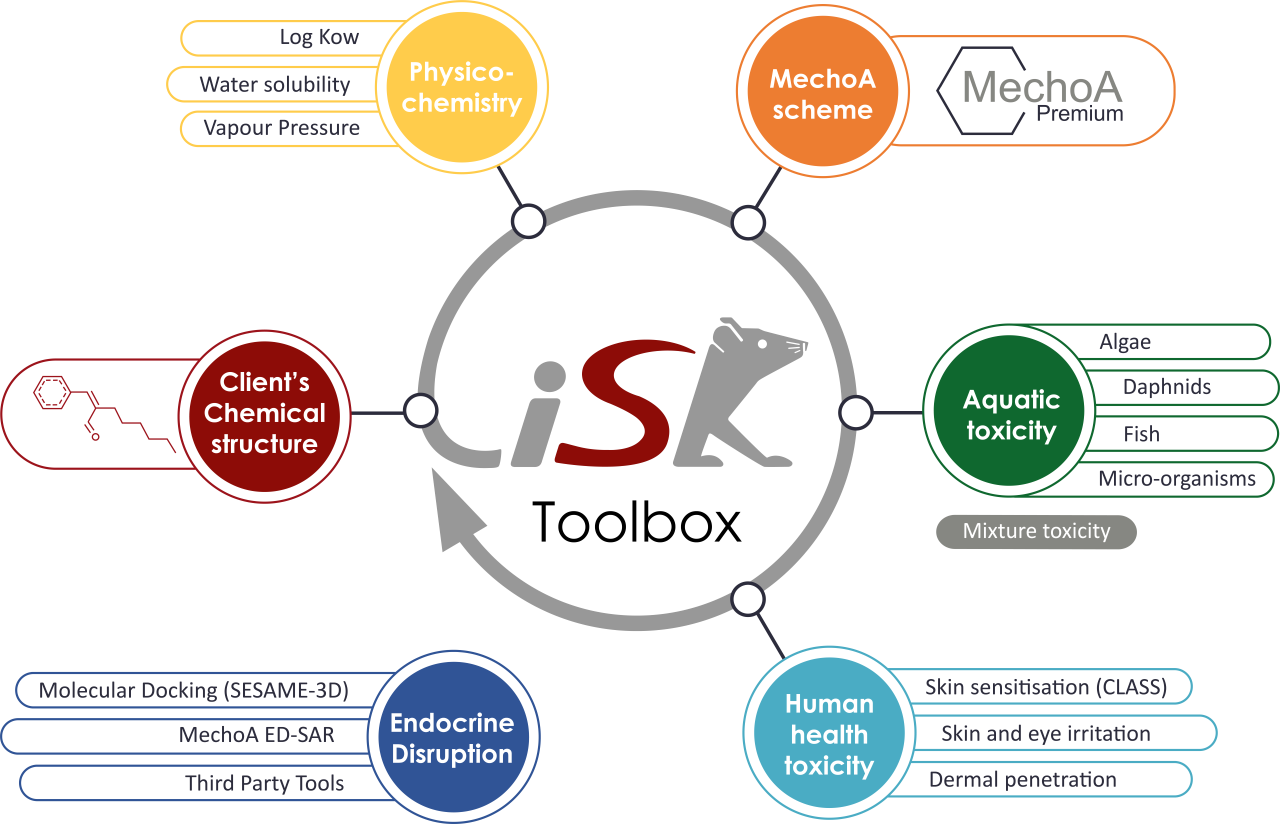
Focus on our iSafeRat® Toolbox
The end of the Summer break has arrived! And with it another opportunity to review the fundamental principles used at KREATiS and incorporated in our iSafeRat Toolbox, designed to meet the REACH requirements to fulfil endpoints for regulatory dossiers.
POSTED 2024-09-05
MORE DETAILS

Dr Marie Darracq--Ghitalla-Ciock will be representing KREATiS at the EUROTOX2024 congress on 8-11 september in Copenhagen
Marie will be presenting the poster: "Classification & Labelling Assessment for Skin Sensitisation (CLASS) update : a mechanistic in silico model to predict skin sensitisation potential"
POSTED 2024-09-03
MORE DETAILS

Working in compliance with Good Modelling Practise (GMoP)
Back to work as the end of the holiday period approaches! At KREATiS, that means following new challenges as QSAR producers, working in compliance with Good Modelling Practise (GMoP).
POSTED 2024-08-29
MORE DETAILS
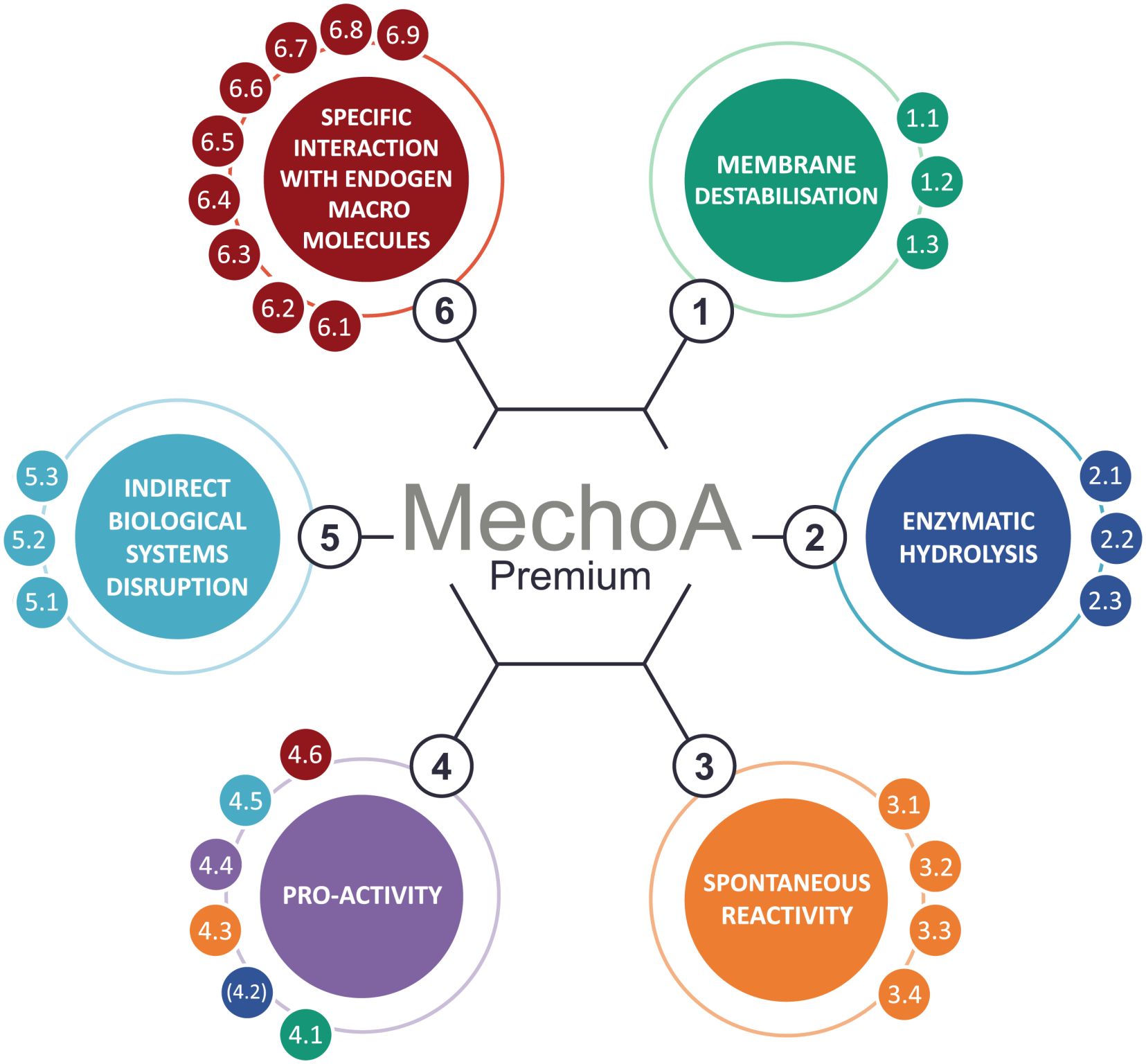
Focus on our freeware MechoApedia
POSTED 2024-08-27
MORE DETAILS

Paul Thomas anime la formation Innovalliance en novembre
Le Dr Paul Thomas PhD ERT, notre expert écotoxicologue, sera un des animateurs de la formation "Biodégradabilité et écotoxicité de vos ingrédients naturels, où en êtes-vous ?"' organisée par Innov'Alliance
POSTED 2024-08-20
MORE DETAILS

Dr Paul Thomas PhD ERT has been accepted as one of the members of the SETAC Vienna Programme Committee
SETAC session proposals: We are delighted to announce that Dr Paul Thomas PhD ERT has been accepted as one of the members of theSETAC Vienna Programme Committee.
POSTED 2024-08-13
MORE DETAILS

Dr Floriane Larras, senior ecotoxicologist and model developer, specialised in biostatistics and Dr Emel Ay-Albrecht, Senior organo-chemist specialised in Artificial Intelligence, take some time off for a well-earned rest and recreation period to visit some little known jewels in Alsace before gearing up for a high energy finish to the year at KREATiS full of new and exciting challenges !
POSTED 2024-08-06
MORE DETAILS

Replay COSMEDTV : l'évaluation des interactions contenant/contenu en cosmétique
Avez-vous vu le dernier hashtag#COSMEDTV par l'Association COSMED ? Carole CHARMEAU-GENEVOIS en était l'invitée pour expliquer les travaux du consortium hashtag#Cosmebooste sur l'évaluation des interactions contenant/contenu en cosmétique.
POSTED 2024-08-01
MORE DETAILS

How do we work in compliance with Good Modelling Practise ?
Paul Thomas PhD ERT explains in this extract of a lecture our High Accuracy-QSAR workflow that explains how we maintain the quality of our work in KREATiS as QSAR producers.
POSTED 2024-07-29
MORE DETAILS

The KREATiS Team wishes you a happy summer season
POSTED 2024-07-24
MORE DETAILS

Future update (v4.3) of iSafeRat® Desktop
POSTED 2024-07-21
MORE DETAILS

The SafePAC application is coming
POSTED 2024-07-20
MORE DETAILS

KREATiS in AromaDays
Last week, we were in Avignon (France) for the hashtag#AromaDays congress by Association COSMED, to share our expertise as (eco)toxicologist specialists about the essential oils.
POSTED 2024-07-15
MORE DETAILS

Participate to our training : "Introduction to in Silico Modeling Approaches for Regulatory Ecotoxicological Hazard Assessment"
Proposed in collaboration with Pr. Mark Cronin from Liverpool John Moores University
POSTED 2024-06-01
MORE DETAILS

Green Swans countering chemical pollution
How can the science on chemical pollution be best developed and translated towards effective utility?
POSTED 2024-05-20
MORE DETAILS

KREATiS aux Quarks Safety Days
Nous avons eu l'opportunité de participer aux QSD de Quarks Safety à l'occasion de notre conférence "Ecotoxicité et santé humaine", présentée par notre experte toxicologue Carole CHARMEAU-GENEVOIS et notre expert écotoxicologue Paul Thomas PhD ERT.
POSTED 2024-05-15
MORE DETAILS

KREATiS participates in another step towards a NAMs based regulatory landscape
Our ecotoxicologist expert Paul Thomas PhD ERT participated at the EPAA hashtag#Designathon hosted by EU Science, Research and Innovation in Italy.
POSTED 2024-04-15
MORE DETAILS

KREATiS presents SafePAC at PCD congress
We had the opportunity to present KREATiS's work in the CosmetoPack® consortium at the Paris Packaging Week in January, alongside Berry Global, Inc., EquiTox, LVMH RECHERCHE and ArGil sas.
POSTED 2024-01-18
MORE DETAILS

Welcome Marie !
We are delighted to welcome Dr Marie Darracq--Ghitalla-Ciock in our team.
POSTED 2024-01-15
MORE DETAILS

KREATiS recognised in the Australian Industrial Chemicals Introduction Scheme
POSTED 2023-12-15
MORE DETAILS

KREATiS tackles biodegradability assessment of complex mixtures
POSTED 2023-07-10
MORE DETAILS

Welcome Jora !
POSTED 2023-03-30
MORE DETAILS

Welcome Claire !
POSTED 2023-03-22
MORE DETAILS

KREATiS present at the #Congrès Parfums Cosmétiques at Chartrexpo
POSTED 2022-11-18
MORE DETAILS

Training course on in silico tools to students of the Master’s 2 Degree TES from the University of Paris City
POSTED 2022-11-15
MORE DETAILS

KREATiS present at 21th edition of ESTIV in Sitges (Barcelona, Spain)
POSTED 2022-11-15
MORE DETAILS
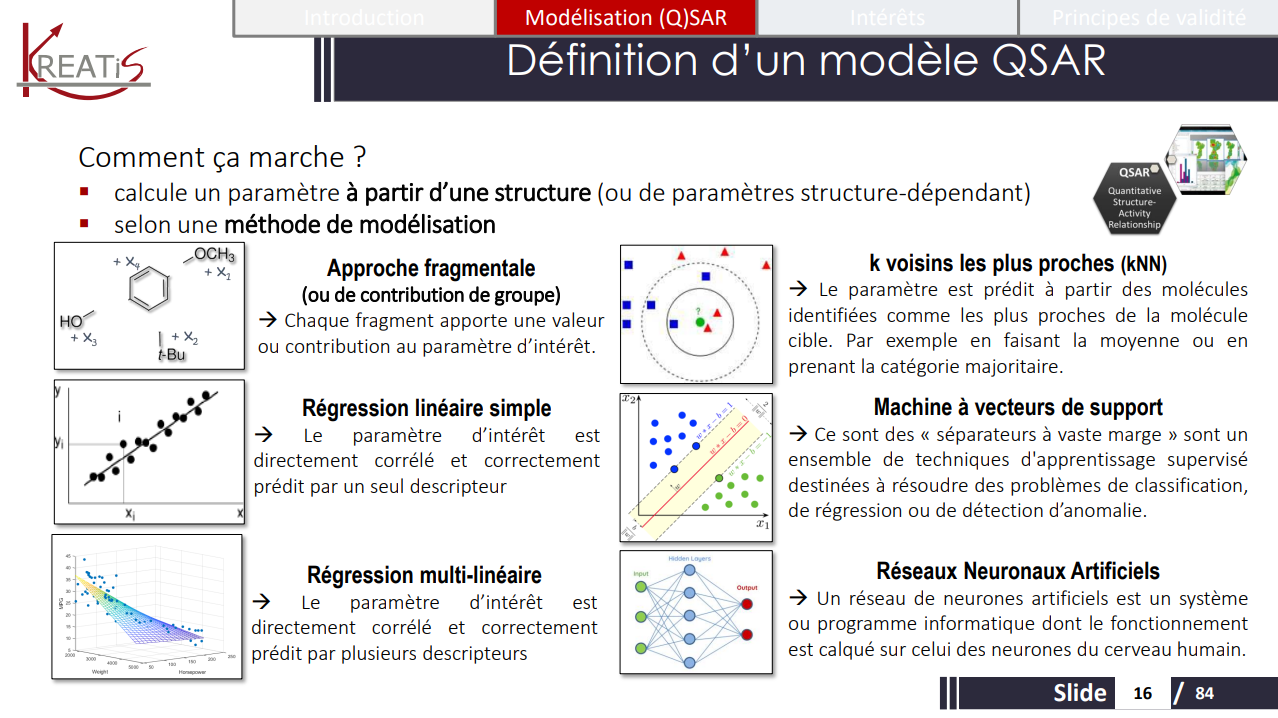
Lesson of the Master's 2 Degree TES of Paris City : " introduction to (Q)SARs"
POSTED 2022-11-08
MORE DETAILS

The ICT congress on toxicology - Feedback
POSTED 2022-10-04
MORE DETAILS

KREATiS at the International Congress of Toxicology in Maastricht
POSTED 2022-09-20
MORE DETAILS

KREATiS at the #International #Congress of #Toxicology ( ICT2022) in Maastricht
POSTED 2022-09-09
MORE DETAILS

A scheme to evaluate structural alerts to predict toxicity
POSTED 2022-09-02
MORE DETAILS

GoodBye Pascal !
POSTED 2022-09-01
MORE DETAILS

Welcome Floriane !
POSTED 2022-06-08
MORE DETAILS

SETAC Europe 32nd Annual Meeting
POSTED 2022-05-18
MORE DETAILS

SETAC sustainable chemicals workshop
POSTED 2022-05-17
MORE DETAILS

SETAC Copenhagen
POSTED 2022-05-12
MORE DETAILS

Welcome Joris !
POSTED 2022-05-05
MORE DETAILS

Welcome Haythem !
POSTED 2022-04-07
MORE DETAILS

Welcome Julie !
POSTED 2022-04-05
MORE DETAILS
Science approach document - ecological risk classification of organic substances version 2.0 (ERC2)
POSTED 2022-03-30
MORE DETAILS

Welcome to Etienne at KREATiS !
POSTED 2022-03-29
MORE DETAILS

Strengthening weight of evidence for fish embryo toxicity data to replace fish acute toxicity tests
POSTED 2022-03-24
MORE DETAILS

Qualiopi Certification
POSTED 2022-03-24
MORE DETAILS

Mechanisms of action (MechoAs): In what way is your substance toxic ?
POSTED 2022-03-03
MORE DETAILS

iSafeRat® platform logo
POSTED 2022-02-24
MORE DETAILS

KREATiS Christmas 2021 words
POSTED 2021-12-21
MORE DETAILS


SETAC Europe 32nd Annual Meeting 15-19 May 2022
POSTED 2021-11-24
MORE DETAILS

KREATiS Newsletter-October 2021
POSTED 2021-11-10
MORE DETAILS

KREATiS announces partnership with Bayer for Advanced Toxicology Testing Software, iSafeRat®
The new technology provides in silico ecotoxicology predictions based on the Mechanisms of Toxic Action (MechOA) decision tree boosting R&D in a time and cost-efficient way
POSTED 2021-11-09
MORE DETAILS
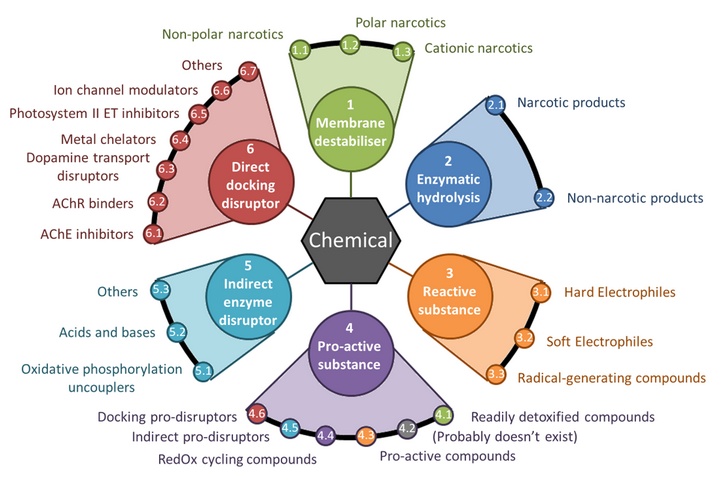
MechoA profiler for OECD QSAR Toolbox is released !
MechoA profiler is now available as a (free) downloadable add-in to the OECD QSAR Toolbox !
POSTED 2021-10-18
MORE DETAILS

News collaboration Unilever
ANNOUNCEMENT OF COLLABORATION BETWEEN KREATiS, UNILEVER & LIVERPOOL JOHN MOORES UNIVERSITY
POSTED 2021-08-30
MORE DETAILS
